Rapid Tests
Cirrus Ag Test
Cirrus COVID-19 Antigen Test is intended to be used for the qualitative detection of SARS-CoV-2 nucleocapsid antigen in nasopharyngeal and oropharyngeal swab specimens from patients suspected of COVID-19.
The antigen can generally be detected in swab specimens taken during the acute phase of the infection. Positive results indicate that nucleocapsid antigen of SARS-CoV-2 was detected, however, it is necessary to check clinical correlation with the patient’s history and other diagnostic findings to determine the infection status. Positive results do not rule out bacterial infections and or co-infection with other viruses. The antigen detected may not be the definite cause of the disease.
Negative results do not rule out SARS-CoV-2 infection and a negative result should not be used as the sole basis for treatment, patient management, and infection control measures. In case of a negative result, a patient's recent exposures, history, and clinical findings consistent with COVID-19 should be taken into account and the result should be confirmed with a molecular test (for example RT-PCR).
The test employs a monoclonal antibody pair raised against the nucleocapsid antigen of SARS-CoV-2. The capture antibody is immobilized on the nitrocellulose membrane and the detection antibody was coupled to colored microparticles and stabilized on a conjugate pad. The swab sample is treated with the extraction solution and then the extracted sample is applied to the sample pad. As the sample migrates forward, if the SARS-CoV-2 nucleocapsid antigen is present, the antigen interacts with the detector antibody and then the complex is captured on the test line. A colored line (T) is visible indicating a positive result. A control line (C) is used for procedural control and it should always appear if the test procedure is performed properly.
Cirrus COVID-19 Antigen Test is an in vitro diagnostic medical device (IVD) intended for professional use. The test should be used by trained clinical laboratory personnel.

Cirrus COVID-19 IgM/IgG Antibody Test is a rapid and qualitative immunochromatographic in vitro assay for differential detection of IgM and IgG antibodies to SARS-CoV-2 in human serum, plasma, and whole blood samples.
The device is designed to aid in the determination of recent or previous exposure to the SARS-CoV-2 virus, tracking the body’s immunity status after infection by the SARS-CoV-2 virus. It only provides a preliminary result. Negative results do not exclude SARS-CoV-2 infection. Patient management decisions should combine negative results with clinical observations, patient history, and epidemiological information.
A positive result does not necessarily mean a current infection but represents a different stage of the disease after infection. Current infection should be confirmed by Real-Time Reverse Transcriptase (RT-PCR) or viral gene sequencing.
When the sample (human serum, plasma, or whole blood) is added to the sample well, the specific antibodies, if any in the sample, will react with the SARS-CoV-2 specific antigens conjugated to colloidal gold. As the immunocomplex migrates along the strip, IgM and IgG antibodies are captured on the respective areas where anti-human IgM and IgG antibodies were immobilized and red/violet lines appear, indicating positive results. A colored procedure control line always forms at the end of the test are
a marked “C”. If SARS-CoV-2 antibodies are absent in the sample, no red/violet line will form in the test lines (G and M), indicating a negative result.
The test is in vitro diagnostic (IVD) device for professional use only.

SARS-CoV-2 Antigen Rapid Test Kit is an in vitro diagnostic rapid test for the qualitative detection of SARS-CoV-2 antigen (Ag) in human nasopharyngeal swab specimens from individuals who meet Covid-19 clinical criteria.
SARS-CoV-2 Antigen Rapid Test Kit detects current infection during the acute phase of Covid-19, while the coronavirus is still present in large quantities in the respitory tract (nasopharynx).
RapidFor ™ Covid-19 Antigen Rapid Test Kit is for professional and home use is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection.
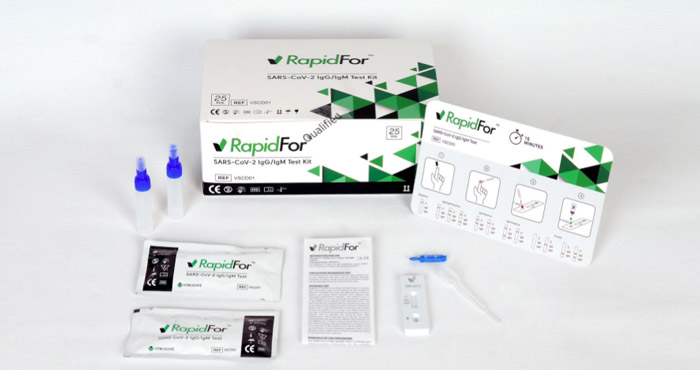
RapidFor® Covid-19 IgG/IgM Rapid Test Kit is a rapid chromatographic immunoassay for the qualitative detection of IgG and IgM antibodies to SARS-CoV-2. This test kit detects the presence of IgG and IgM antibodies in serum, plasma and whole blood samples.
Antibody Rapid Test Kit detects whether you had COVID-19 in the past and now have antibodies against the virus. Antibodies are proteins made by the immune system to fight infections like SARS-CoV-2. Antibodies can take days or weeks to develop in the body following exposure to a SARS-CoV-2 (COVID-19) infection. IgM antibody is the earliest antibodies in Covid-19 infection. IgG antibodies appears later in the blood. IgG antibodies stay in your blood long after the covid infection goes away.